ICOD for Down syndrome families
Down syndrome is the most common chromosomal abnormality in children leading to lifelong intellectual disability.
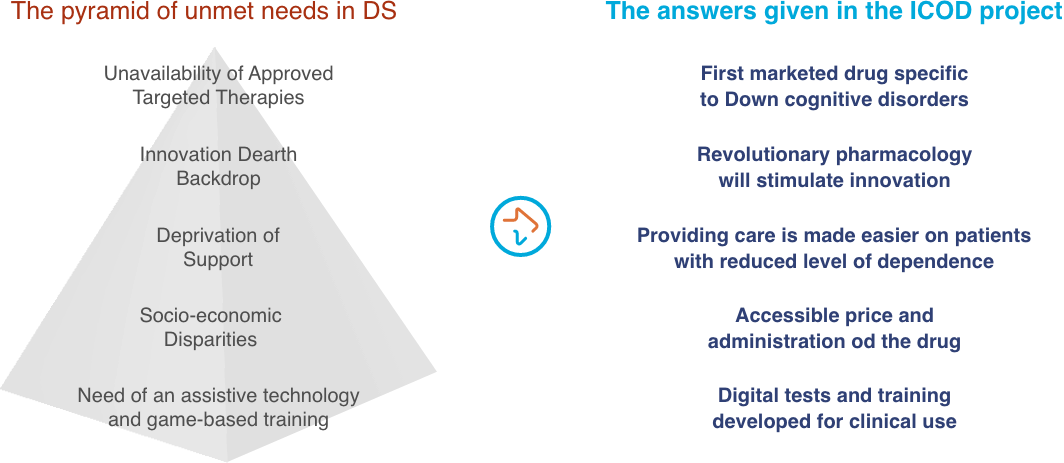
The increase in life expectancy of subjects with Down syndrome has confronted families with the very challenging problem of how to take care of a disabled child that will survive them. Their cognitive impairment is one of the main preoccupations of their families, as it leads to a profound dependence, reduced quality of life and social stigma.1,2
Despite the heavier social burden and the greater medical needs linked to this intellectual disability, there is no an approved therapy for Down syndrome-related cognitive impairment yet.
Can the ICOD project change this scenario and the life of Down syndrome families?
The ICOD project is expected have a strong impact on Down syndrome families for two reasons:
- The ICOD project will test a first in class drug, AEF0217, developed specifically to improve cognitive performance and adaptive functionality in individuals with Down syndrome.
- The ICOD project will use a novel psychometric approach able to better evaluate cognitive impairment and the potential benefit of AEF0217.
How will the ICOD project test AEF0217 in subjects with Down syndrome?
The ICOD project aims to perform “first in human” trials with AEF0217 to test:
- The safety, tolerability and pharmacokinetic profile (plasma concentration of the drug) of AEF0217 in Phase I trials in healthy volunteers and in individuals with Down syndrome. The Down syndrome population is quite fragile, and families are strongly preoccupied by the potential side effects of new treatments. The preclinical AEF0217 studies do not predict any side effects on behavior and significant toxicity, fulfilling the safety concerns of families of subjects with Down syndrome.
- The efficacy of AEF0217 on cognitive impairment in Down syndrome in a Phase II trial once the safety and tolerability Phase I studies are completed in healthy volunteers and in individuals with Down syndrome. A total of 128 young adults (18-35 years) with Down syndrome will participate in an international multicenter study (Italy, France and Spain). The goal of a pharmacological treatment for cognitive impairment in Down syndrome is to restore previous impaired learning mechanisms. Consequently, results can be biased by differences in their training which are generally not addressed in clinical trials in intellectual disabilities. For this reason, the clinical trial will incorporate a standardized cognitive training: all volunteers will receive cognitive training.
The ICOD project will evaluate cognitive function with NIH Toolbox1 but also patient-reported quality of life (QoL) measures and caregiver-based evaluation.
Three months of treatment with AEF0217 are expected to bring improvement on working memory enough to significantly gain in learning skills and consequently in autonomy, in quality of life and therefore reduce health care costs.
Down syndrome families’ associations and organizations have tried quite successfully to provide support to families in different issues (i.e. job, sexuality, health care), assuming there would not be therapeutic opportunities to treat cognitive impairment in intellectual disability. Breaking this glass ceiling is our goal. We hope that the ICOD project will bring this revolution of the first effective treatment for cognitive impairment.
The impact of the ICOD project is aligned with the objectives of the European Disability Strategy 2010-20, aiming at eliminating barriers linked to the accessibility, participation, employment, education, training, social protection and health of people with Down syndrome.
References
1. Shields RH, et al. Validation of the NIH Toolbox Cognitive Battery in intellectual disability. Neurology. 2020 Feb 24. pii: 10.1212/WNL.0000000000009131.
2. Antón JI, et al. An analysis of the cost of disability across Europe using the standard of living approach. SERIEs, 2016, 7(3) 281–306.